Empowering Breast Cancer Patients with Genetic Testing
In 2013, Academy Award-winning actress Angelina Jolie wrote a now famous opinion piece for the New York Times detailing her journey involving genetic testing for breast and ovarian cancer.
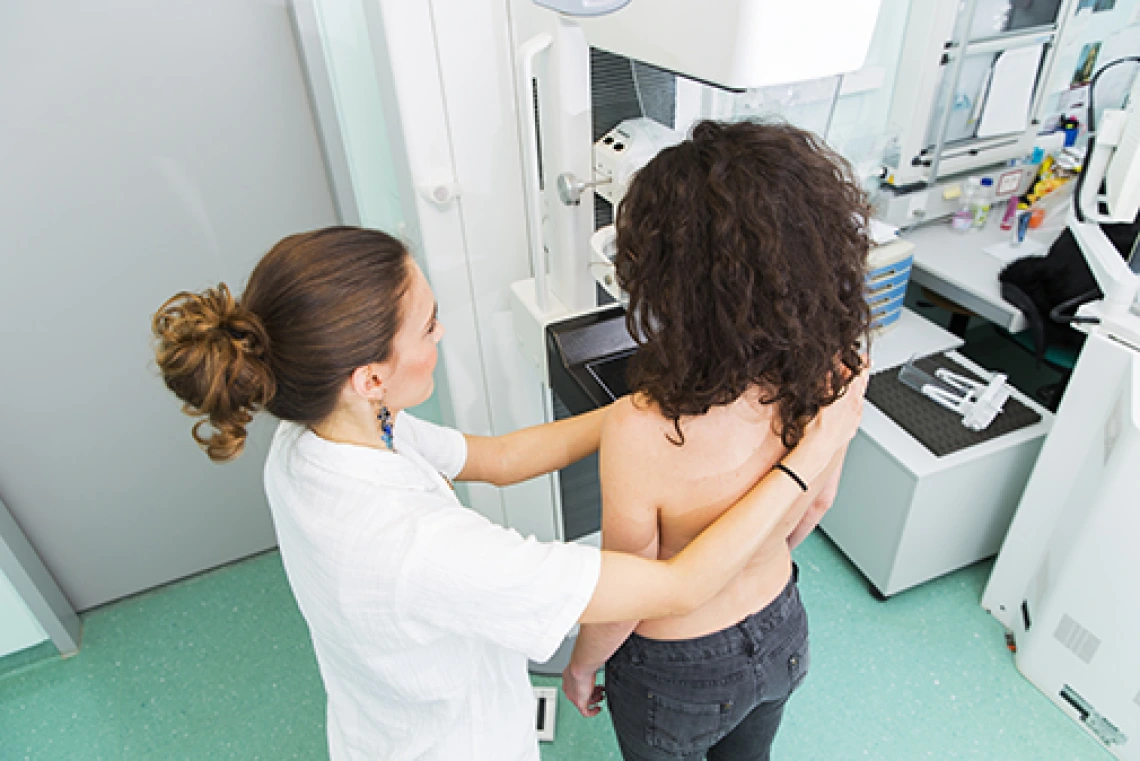

A woman with a disease-causing mutation in BRCA1 or BRCA2 has a 69 – 72 percent chance of developing breast cancer by age 80.
Jolie worked with her doctor to undergo genetic testing for several different genes that, when they mutate, increase a woman’s risk of developing breast cancer and ovarian cancer.
The two most commonly mutated genes associated with breast cancer are BRCA1 and BRCA2. BRCA stands for BReast CAncer susceptibility gene. First discovered in 1990, BRCA1, like its cousin BRCA2, codes for a protein that helps repair damaged DNA inside the cell. When someone inherits a faulty copy of BRCA1 or BRCA2, their body cannot repair damaged DNA as efficiently, and this eventually can lead to certain forms of cancer.
Diagnostic genetic testing, which can tell more about whether you have a mutation in your BRCA1 or BRCA2 genes, is becoming more accessible as the technology to sequence DNA continues to improve and become less expensive.
Mutations in the BRCA1 and BRCA2 genes not only increase the risk of developing cancer, the cancer also tends to strike people at a younger age. In addition, men with a mutation in BRCA1 or BRCA2 are at increased risk of developing breast cancer and prostate cancer.
The two most commonly mutated genes associated with breast cancer are BRCA1 and BRCA2. BRCA stands for BReast CAncer susceptibility gene.
Diagnostic genetic testing, which can tell more about whether you have a mutation in your BRCA1 or BRCA2 genes, is becoming more accessible as the technology to sequence DNA continues to improve and become less expensive. Talking with a primary care doctor or gynecologist, combined with guidance from a genetic counselor to discuss your family history of cancer, can help determine if genetic testing is the right decision for you.

Talking with a primary care doctor or gynecologist, combined with guidance from a genetic counselor to discuss your family history of cancer, can help determine if genetic testing is the right decision for you.
About the Author
Valerie Schaibley, PhD is the Administrator for the Center for Applied Genetics and Genomic Medicine at the University of Arizona Health Sciences, where she works to advance precision health in the state of Arizona. She received her PhD in Human Genetics from the University of Michigan and worked for several years in industry, developing genetic tests for precision medicine applications.
About the Author
Kenneth S. Ramos, MD, PhD, PharmB, served as associate vice president for precision health sciences at the University of Arizona Health Sciences, director of the Center for Applied Genetics and Genomic Medicine and the MD-PhD Program, and professor of medicine. In 2019, Dr. Ramos accepted a position as executive director of the Institute of Biosciences and Technology in Houston and assistant vice chancellor for Health Services at The Texas A&M University System.
Dr. Ramos is a physician-scientist with interests in molecular and precision medicine, particularly as it relates to vascular pathology, oncology and chronic diseases of the lung. His translational research program integrates diverse approaches ranging from molecular genetics to population-based studies to elucidate genetic and genomic mechanisms of pathogenesis, and to develop novel approaches and therapies to minimize chronic diseases caused by environmental injury. Ongoing translational studies in his laboratory focus on the study of repetitive genetic elements in the mammalian genome and their role in genome plasticity, toxicity and disease, while clinical studies focus on the development and characterization of diagnostic and prognostic biomarkers of cancer and chronic pulmonary disease to advance the goals of personalized genomic medicine. He has directed two NIH P30 Centers of Excellence working at the interface between genomics and environmental health and medicine and have provided administrative and scientific leadership for two academic centers focusing on genetics and genomic medicine. He has influenced the career of many scientists through my involvement in several NIH-funded training and career development programs where he has mentored over 100 doctoral, medical, veterinary, undergraduate and high school students, many of whom have gone on to successful careers in academia, medicine, government and industry. He is deeply committed to initiatives that advance precision medicine and its applications to reduce disease burden and health disparities, improve quality of healthcare and reduce costs.

