Health Sciences Researchers Close In on Diabetes Solution
Hunting for decades, Drs. Klearchos Papas, Robert Johnson and Tom Loudovaris help develop a bio-artificial pancreas at UArizona Health Sciences.

Klearchos Papas, PhD, director of the College of Medicine – Tucson’s Institute for Cellular Transplantation, holds a synthetic nanoporous pouch used in an implantable cell therapy device to provide insulin for Type 1 diabetes patients.
Home from college at age 19, Robert Johnson, PhD, couldn’t seem to quench his thirst.
“I would wake up in the night, run into the bathroom, stick my head under the faucet and drink, drink, drink,” said Dr. Johnson, a Department of Surgery research professor at the College of Medicine – Tucson. “I would have dreams I was crawling across the Sahara Desert on my elbows. It was so dry.”

Robert C. Johnson, PhD, is a research professor in the College of Medicine – Tucson’s Institute for Cellular Transplantation.
“It was between Christmas and New Year’s Day, and I wanted to have fun with my friends,” Dr. Johnson said. “I didn’t want to go to the hospital. And the doctor said to me, ‘Well, Robert, you can either come in now, or we’ll bring you in feet first in a few days.’ That caught my attention.”
For Tom Loudovaris, PhD, also a research professor in the Department of Surgery, his 9-year-old son’s hernia was his wake-up call about Type 1 diabetes. At the doctor’s office, a nurse suggested his son take a glucose test. That test showed his son, now in his 20s, had the chronic health condition. His daughter later showed the classic symptoms – thirst, hunger, frequent urination, fatigue, mood shifts and blurred vision – for Type 1 diabetes. She tested positive, too.
Today, Drs. Johnson and Loudovaris are part of a University of Arizona Health Sciences research team seeking to develop a cell encapsulation device as a solution for Type 1 diabetes. The team is led by Klearchos Papas, PhD, professor and director of the Institute for Cellular Transplantation in the Department of Surgery at the College of Medicine – Tucson.
The team’s solution is composed of islet cells protected in a special oxygen-enabled pouch implanted to supply insulin the body needs and control blood sugar levels without immunosuppressants, or anti-rejection drugs. It’s essentially a bio-artificial pancreas.

Tom Loudovaris, PhD, is a research professor in the College of Medicine – Tucson’s Institute for Cellular Transplantation.
Between the three of them, Drs. Papas, Johnson and Loudovaris have about 100 years of research experience with Type 1 diabetes. Their novel therapy may be several years away from full regulatory approval, but the research team has made rapid progress toward the goal courtesy of their advanced oxygen-enabled implantable pouch, which shows promise for delivery of other disease remedies as well. Additional applications include conditions such as hemophilia, adrenal deficiency, and immunotherapy in cancer.
Still, Type 1 diabetes treatment shows the most immediate potential. It’s something Dr. Papas has been working on for well over two decades with funding from the Juvenile Diabetes Research Foundation, National Institute of Diabetes and Digestive and Kidney Diseases, and now Novo Nordisk A/S, a Danish global pharmaceutical company.
In December, his firm Procyon Technologies LLC signed an agreement with Novo Nordisk A/S to further develop the invention with the company’s stem cell-derived insulin-secreting cells. Procyon licensed the cellular implantation technology through Tech Launch Arizona, UArizona’s commercialization arm.
Dr. Johnson said he’s excited to continue working on research he pioneered earlier in his career as research director of Baxter Healthcare’s Cell and Gene Therapy Unit. That group, later spun off by Baxter as TheraCyte Inc. under Dr. Loudovaris’ leadership, created an early version of the synthetic membrane-based implantable pouch. The uniqueness of the UArizona Health Sciences effort revolves around its pairing of a nanoporous pouch with an oxygen delivery system engineered by Dr. Papas to sustain Novo Nordisk’s insulin-producing stem cells.

Klearchos Papas, PhD, director of the College of Medicine – Tucson’s Institute for Cellular Transplantation, in his lab.
Dr. Johnson joined the College of Medicine – Tucson’s faculty four years ago after coming out of semi-retirement from Baylor College of Medicine, where he had been general manager of its Medical Genetics Labs. Dr. Loudovaris had been collaborating with Dr. Papas since 2011 in his other role as manager of the Tom Mandel Islet Transplant Program at the St. Vincent’s Institute of Medical Research in Melbourne, Australia.
Dr. Loudovaris said the ability to dispense with immunosuppressives with the team’s implanted oxygen-enabled encapsulation device is significant.
“These drugs have a big impact on your life,” he said. “You have to maintain the immune suppression constantly. We have transplant recipients who have been insulin independent for over 10 years, who’ve begun to show the negative impact of the immunosuppressive drugs. We don’t want them to get kidney failure, but we also don’t want them to become diabetic again – tough decisions may have to be made.”
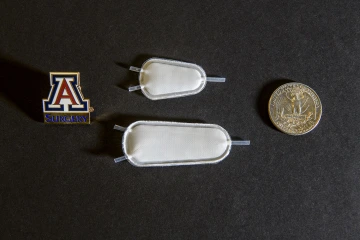
Two samples of a synthetic implantable pouch, which would be infused with insulin-producing cells as a therapy for Type 1 diabetics.
The oxygen-enabled implantable cell therapy pouch differs from an insulin pump, Dr. Papas said, in that it will closely mimic a normal pancreas and offer more durable, timely and accurate glucose sensing and insulin delivery than the delayed response of an external pump and glucose sensor.
This is because, as vasculature develops around the implanted pouch, contained islet cells take their cues from the body’s natural biochemistry to sense glucose and deliver insulin as needed more immediately and precisely – just as normal cells in the pancreas would.
Dr. Papas, who also is a member of the BIO5 Institute, said he has long admired Drs. Johnson and Loudovaris’ work on Type 1 diabetes.
“They were very successful at Baxter in the ’80s and ’90s,” he said. “They actually did some clinical work in Sweden and showed they could protect cells from the immune system. They created a pouch that blocked it but, essentially, they didn’t have the islet cells or oxygen delivery yet. They were way ahead of their time.”
All three researchers hope to begin human clinical trials within three years.

