Tracking Down the Roots of Childhood–Onset Epilepsy
Epilepsy is the fourth most-common neurological disorder in adults, and the most common neurological condition in children. About 1 in 26 people will develop epilepsy over the course of their lifetime.
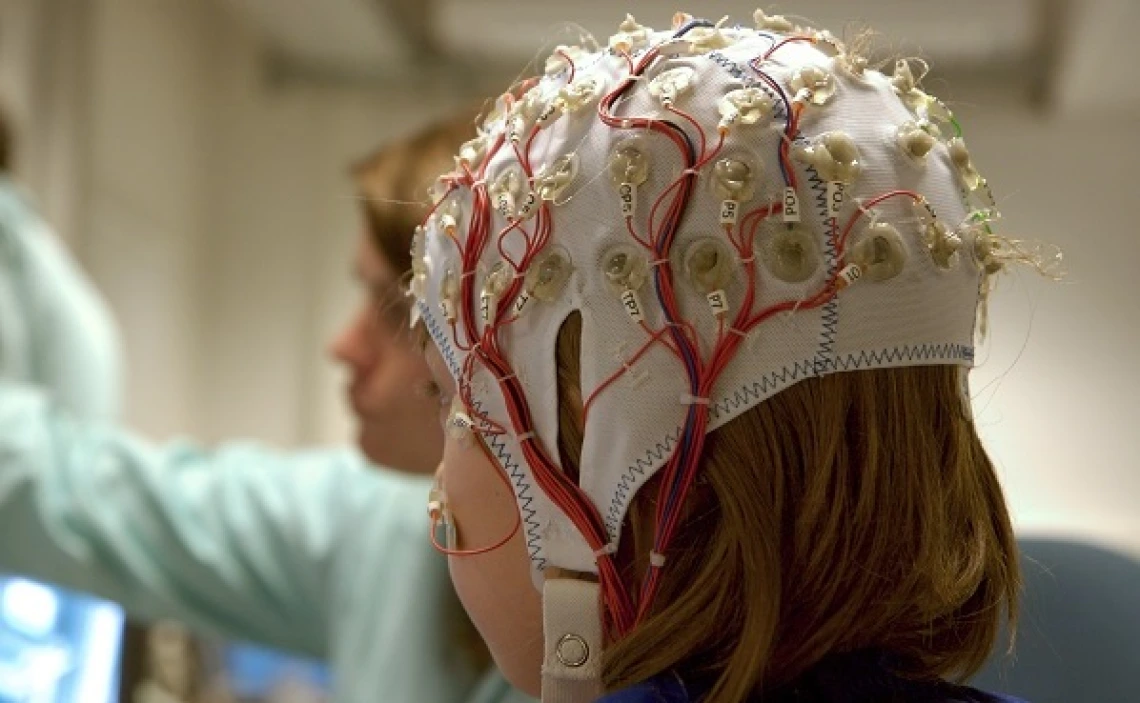
People with epilepsy have disrupted patterns of neuronal activity that can cause strange sensations, emotions and behavior. Sometimes, these disruptions in neuronal activity can lead to convulsions, muscle spasms and loss of consciousness. In adults, epilepsy most often is caused by traumatic brain injury, infection, tumors or other neurological conditions. On the other hand, epilepsy in children more often directly is related to genetics.
Michael Hammer, PhD, research scientist with the Arizona Research Labs, director of the University of Arizona Genetics Core, and director of the Technology and Innovation Division of the Center for Applied Genetics and Genomic Medicine, built his career studying human population genetics. For years, his laboratory analyzed the ways evolution affects the patterns of genetic variation that we see throughout the human genome.

Epilepsy in children is more often directly related to genetics.
In 2012, Dr. Hammer’s laboratory found the mutation that caused his daughter’s epilepsy: a single change in the DNA sequence in a gene called SCN8A. This was the first reported case of epilepsy caused by a mutation in the SCN8A gene.
The SCN8A gene codes for one part of a large protein complex known as the sodium channel. These channels control how sodium atoms in the human body flow in and out of cells. Sodium channels are in all cells of the body, including cells of the brain. In neurons, sodium channels regulate how cells communicate with each other, and specifically control the generation and transmission of electrical signals in the nervous system.
Changes in the sequence of the SCN8A gene can change the function of the sodium channel. People with SCN8A-related epilepsy have mutations that increase the flow of sodium ions into nerve cells, leading to an over-excited state in the brain and uncontrolled electrical signals. This hyperactive state of neurons leads to abnormal brain function and epilepsy.

After initially discovering the SCN8A mutation in his daughter, Dr. Michael Hammer, started reaching out to other families of children with epilepsy in order to identify the underlying genetic causes of childhood-onset epilepsy on a more global scale.
Dr. Hammer joined with other families who learned that their child had a variant in SCN8A to initiate a support group, and he also manages a community website for people facing similar struggles. This website also hosts a registry, which gathers data from people with SCN8A mutations from around the world (http://scn8a.net). Today, his website and registry have collected data from more than 200 families of children with SCN8A-related epilepsy, yielding important information for families, clinicians and researchers.
Dr. Hammer’s work now focuses on finding treatments to improve the symptoms and quality of life for people with epilepsy.
“Everyone is somewhat susceptible to seizures, but different people have different thresholds to overcome. Some people have a high threshold that makes it more difficult for them to get to the point of having a seizure. Others have a lower threshold where seizures happen more easily,” says Dr. Hammer. “People with epilepsy have an extremely low threshold, and therefore, they have seizures more frequently.”

A child with epilepsy during a seizure.
For more information about events supporting epilepsy, please go to http://www.purpleday.org/events. To learn more about SCN8A-related epilepsy, please visit http://scn8a.net.
About the Author
Valerie Schaibley, PhD is the Administrator for the Center for Applied Genetics and Genomic Medicine at the University of Arizona Health Sciences, where she works to advance precision health in the state of Arizona. She received her PhD in Human Genetics from the University of Michigan and worked for several years in industry, developing genetic tests for precision medicine applications.
About the Author
Kenneth S. Ramos, MD, PhD, PharmB, served as associate vice president for precision health sciences at the University of Arizona Health Sciences, director of the Center for Applied Genetics and Genomic Medicine and the MD-PhD Program, and professor of medicine. In 2019, Dr. Ramos accepted a position as executive director of the Institute of Biosciences and Technology in Houston and assistant vice chancellor for Health Services at The Texas A&M University System.
Dr. Ramos is a physician-scientist with interests in molecular and precision medicine, particularly as it relates to vascular pathology, oncology and chronic diseases of the lung. His translational research program integrates diverse approaches ranging from molecular genetics to population-based studies to elucidate genetic and genomic mechanisms of pathogenesis, and to develop novel approaches and therapies to minimize chronic diseases caused by environmental injury. Ongoing translational studies in his laboratory focus on the study of repetitive genetic elements in the mammalian genome and their role in genome plasticity, toxicity and disease, while clinical studies focus on the development and characterization of diagnostic and prognostic biomarkers of cancer and chronic pulmonary disease to advance the goals of personalized genomic medicine. He has directed two NIH P30 Centers of Excellence working at the interface between genomics and environmental health and medicine and have provided administrative and scientific leadership for two academic centers focusing on genetics and genomic medicine. He has influenced the career of many scientists through my involvement in several NIH-funded training and career development programs where he has mentored over 100 doctoral, medical, veterinary, undergraduate and high school students, many of whom have gone on to successful careers in academia, medicine, government and industry. He is deeply committed to initiatives that advance precision medicine and its applications to reduce disease burden and health disparities, improve quality of healthcare and reduce costs.

