New Safeguards Allow Cancer Care, Research to Continue
UArizona Cancer Center, Banner – University Medicine continue state-of-the-art cancer care and cutting-edge research during COVID-19 pandemic

The University of Arizona Cancer Center created new policies based on what it learned from other states and countries about how to continue cancer care and research during a pandemic.
When it was clear the COVID-19 pandemic would affect the United States, leaders at the University of Arizona Cancer Center and its clinical partner Banner – University Medicine had two critical questions to answer quickly.
First, what must be done to protect patients and staff from the spread of the novel coronavirus? Second, how can essential cancer care and research continue?

Julie E. Bauman, MD, is UArizona Cancer Center deputy director, pictured here examining a patient in 2017.
“Cancer doesn’t sleep, so neither can we,” said Julie E. Bauman, MD, UArizona Cancer Center deputy director. Dr. Bauman is also a professor in the College of Medicine – Tucson Department of Medicine, and chief of the Division of Hematology and Oncology at the college and Banner – University Medicine Tucson.
“We've been working for nearly three months to create and maintain a ‘bubble’ around the Cancer Center so that world-class cancer care and research can continue. That is crucial because we don’t know how long this pandemic will last,” Dr. Bauman said.
Learning from Others
She and her team quickly took actions based on what they learned from those who experienced the spread of COVID-19 earlier in the year.
First, leadership at the Cancer Center and Banner – University Medicine were in constant communication. Multiple daily meetings were scheduled to discuss provider capacity, quantity of supplies, such as personal protective equipment and swabs, and other essential needs to ensure safe operations.
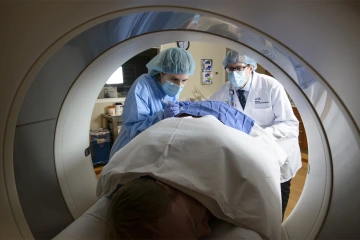
A patient undergoes a CT scan, a way to capture images of a person's organs or skeletal system.
“There's been continuous conversation,” said Sandra Kurtin, PhD, director of advanced practice and clinical integration at the Cancer Center. “Everyone is trying to be transparent about what is happening, what we know, what we don't know and what we can't know. There was a lot that went into decision-making that was very methodical and thoughtfully done. And, I think we actually set a standard across Banner.”
Implementing Clinical and Research Changes
Keeping COVID-19 out of the clinic begins with a comprehensive screening process for all individuals prior to entering the building, including patients, staff and clinicians. Screening includes body temperature checks and a series of questions for symptoms or contacts associated with COVID-19, said Dr. Kurtin, who is the center’s COVID-19 onsite captain.
An on-site drive-through testing process was developed for patients who fail the screening process. It includes medical evaluation and testing for COVID-19. A process was developed for triaging patients who require essential treatments and are under investigation for COVID-19. The triage process limits their exposure to other patients, clinicians and staff. In addition, continuous masking was mandated early for all patients and health care workers.
Medical imaging equipment at the UArizona Cancer Center.
Dr. Bauman agreed, “We know we can’t hold COVID-19 exposure to zero due to community spread, but we are doing everything in our power to be as close to zero as possible. Now three months into the pandemic, the data suggest that our efforts may have minimized the number of patients afflicted with both cancer and COVID-19 compared to similar National Cancer Institute-designated, comprehensive cancer centers.”
Rotating Teams
The next critical step was to ensure health-care providers remained healthy and had minimal exposure threat to COVID-19. During the surge in Arizona, Dr. Bauman implemented a rotating-staff model, comprised of two teams.
The field-based “Team A” members would work for 14 days in the clinic and hospital – critical environments with risk of exposure that have to be staffed nonstop.
Meanwhile, the home-based “Team B” staff would self-quarantine for 14 days and conduct telemedicine services. The 14-day absence, which aligns with the U.S. Centers for Disease Control and Prevention quarantine and active-monitoring period for the virus, enabled a healthy reserve staff to take over clinical care.
“One of the major lessons learned early on from countries like Italy and cities like Seattle and New York is that it is imperative to protect workforce capacity across the service line,” Dr. Bauman said.
“If providers start to become ill or must self-quarantine, we need to be ready with a reserve force that is unexposed.” - Julie E. Bauman, MD, UArizona Cancer Center deputy director
Another measure implemented to maintain a safe clinical setting was to transition many patients to telemedicine rather than in-person visits. In fact, all visits that do not require physical presence in the building have moved to telemedicine.
Essential cancer research is ongoing. Clinical trials continue to accrue patients, especially trials offering new treatments unavailable elsewhere in Arizona. Researchers prioritized essential Cancer Center laboratory projects and implemented safeguards so that scientific discoveries to defeat cancer can continue. This includes social-distancing measures in laboratories and enabling researchers to work together on novel team-science research projects through telephone and Zoom meetings.

