Pandemic Provides New Target for Pain Relief Research
An unexpected finding gives researchers in the UArizona Health Sciences Comprehensive Center for Pain and Addiction a novel target for non-opioid pain therapeutics.
Pain researchers at the University of Arizona Health Sciences are exploring a new way to solve the opioid epidemic thanks to information gleaned from the unlikeliest of sources: the COVID-19 pandemic.

Rajesh Khanna, PhD, led a research team that discovered the SARS-CoV-2 virus promotes pain relief through the receptor neuropilin.
As of late-September, there have been more than 32 million cases of COVID-19 globally and nearly 1 million people have died, according to Johns Hopkins University. In the United States, more than 7 million cases have resulted in over 200,000 deaths.
As scientists around the world work relentlessly to learn more about SARS-CoV-2 – the virus that causes COVID-19 – Comprehensive Center for Pain and Addiction researchers in the College of Medicine – Tucson recently identified a protein at the intersection of the pandemic and an opioid epidemic that may be the gateway to developing new treatments for chronic pain: neuropilin-1.
Following the Pain Pathway
Viruses infect host cells through protein receptors on cell membranes. Early in the pandemic, scientists established that the SARS-CoV-2 spike protein uses the angiotensin-converting enzyme 2 (ACE2) receptor to enter the body. But in June, two papers posted on the preprint server bioRxiv pointed to neuropilin as a second receptor for SARS-CoV-2.
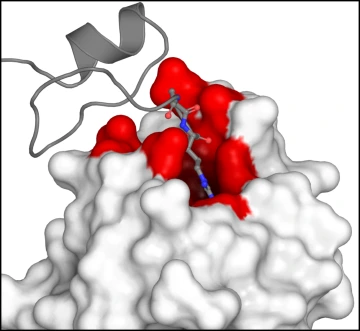
The binding of VEGF-A (grey) to neuropilin (white) happens at a specific site (red) that sets in motion a signaling pathway that leads to pain.
“That caught our eye because for many years my lab has been studying a complex of proteins and pathways that relate to pain processing that are downstream of neuropilin,” said Rajesh Khanna, PhD, a professor in the Department of Pharmacology. “We stepped back and realized this could mean that maybe the spike protein is involved in some sort of pain processing.”
Many biological pathways signal the body to feel pain. One is through a protein named vascular endothelial growth factor-A (VEGF-A), which plays an essential role in blood vessel growth but also has been linked to diseases such as cancer and rheumatoid arthritis.
Like a key in a lock, when VEGF-A binds to the receptor neuropilin, it initiates a cascade of events that affect the activity of key ion channels that are linked to pain. Dr. Khanna has been studying those ion channels – specifically voltage-gated calcium and sodium channels – and the proteins and signaling pathways that activate them for more than 15 years.

Kimberley Gomez, PhD, locates a cell that will be digitally recorded as it is exposed to VEGF-A, SARS-CoV-2 and small molecules.
“At the protein level, the calcium and sodium channels work in concert to change the overall dynamic nature of the sensory neuron, allowing neurons to be excitable,” said Dr. Khanna, who is a member of the university’s BIO5 Institute. “Sodium is responsible for the generation of the excitability, and then calcium drives the changes in the neurotransmitters that are really important for pain. The transmitter is where the message is coded for pain, because that transmitter then acts on neighboring neurons and recruits them into the pain pathway, amplifying their activity.”
Dr. Khanna and his research team found that the SARS-CoV-2 spike protein binds to neuropilin in the same location as VEGF-A, and when it does, the VEGF-induced pain is completely reversed. The paper, “SARS-CoV-2 Spike protein co-opts VEGF-A/Neuropilin-1 receptor signaling to induce analgesia,” was published in PAIN, the journal of the International Association for the Study of Pain.
A New Player in Pain
The findings identified neuropilin as a new target for non-opioid pain relief, a category of therapeutics desperately needed to address the public health crisis created by opioid misuse and abuse.
“For chronic pain, opiates aren’t the best therapy long term, and I think most pain physicians agree with that. It results in things like people abusing the opiates and then becoming addicted,” said Todd Vanderah, PhD, director of the Comprehensive Center for Pain and Addiction and a professor of pharmacology. “At the University of Arizona Health Sciences, we are looking for new medications and therapies that don't produce the addictive quality or euphoric effect of opiates to help people with chronic pain.”
Using an internal recording solution, shown being loaded into a glass electrode, scientists were able to view and document the action of various proteins and small molecules on neuropilin.
Dr. Khanna’s research has already led to the development of small molecules that show potential in blocking the signaling pathways that control channel activity. Working with researchers at Regulonix, a biotechnology company he co-founded, Dr. Khanna has isolated compounds that are non-addictive and more effective than morphine when tested in animal models.
He hopes to find similar success with neuropilin. As part of the SARS-CoV-2 study, Dr. Khanna tested existing small molecule neuropilin inhibitors that were originally developed to suppress tumor growth in certain cancers. Like the SARS-CoV-2 spike protein, they provided the same pain relief when binding to neuropilin. He is currently identifying additional small molecule neuropilin inhibitors to test in future studies.
“Chronic pain is pretty complicated. At the protein level, there are many diverse pathways and proteins that intersect to set baseline pain,” Dr. Khanna said. “We are moving forward with designing small molecules against neuropilin, particularly natural compounds, that could be important for pain relief.”
Our Experts
Contact
Stacy Pigott
Office of Communications
520-621-7239 / 520-539-4152
spigott@arizona.edu

