What it Takes to Create a Vaccine
As COVID-19 vaccine work continues throughout the country, a University of Arizona Health Sciences researcher explains how a vaccine is created.
Public health officials have repeatedly stressed that, until there’s a vaccine for the virus that causes COVID-19, individual and societal measures to reduce its spread need to continue.
To understand the path from pandemic to vaccine, Yin Chen, PhD, associate professor of pharmacology and toxicology in the College of Pharmacy, explained what it takes to train the immune system to fight off a virus. Dr. Chen is not working on a COVID-19 vaccine, but he has spent his career studying the effect of viruses, such as those that cause the common cold and the flu, on the lungs.
An overview of the process

A research scientist analyzes cells infected with a virus in a University of Arizona Health Sciences laboratory.
Generally speaking, vaccine creation can be divided into the developmental stage and the manufacturing stage, Dr. Chen said. At the developmental stage, researchers take the science from the lab, where the virus is examined from every angle, to clinical trials of a vaccine they hope will be approved by the Food and Drug Administration. After FDA approval, a manufacturer, which also may have been involved in the research and trial stages, oversees the production and distribution process.
The goal of a vaccine is to stimulate a response in a healthy person that will generate immunity against a specific pathogen. There are multiple strategies for developing a vaccine for any virus, explained Dr. Chen, who is a member of the University of Arizona’s BIO5 Institute and the College of Pharmacy’s Southwest Environmental Health Sciences Center.
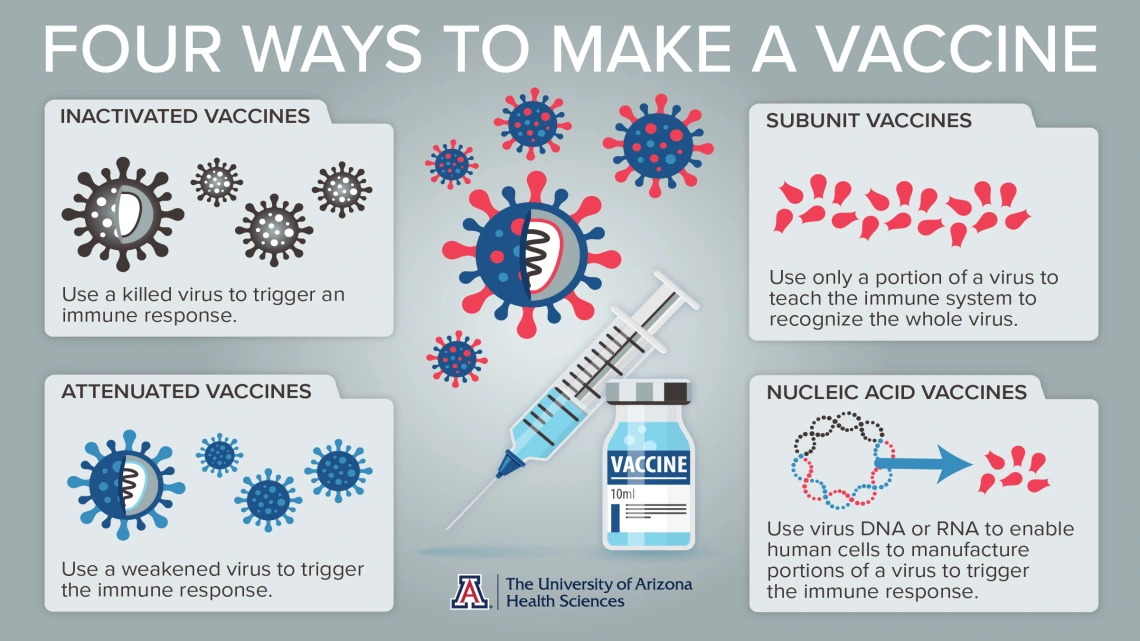
The original vaccine: inactivated or attenuated virus
For the past 100 years, scientists have created vaccines by using pathogens themselves to stimulate immunity. Scientists either kill the virus or weaken it so the recipient won’t have a potentially fatal reaction. These are called inactivated or attenuated vaccines.
The goal is to use that more benign version of the virus to stimulate enough protective immunity to prevent an infection. The immunity comes from the combined forces of different types of lymphocytes, including B cells that secrete antibodies – proteins that target a specific pathogen.
This method of using weakened or killed pathogens is “the archetype of vaccine development,” Dr. Chen said.
Zeroing in on one protein: a subunit of the virus
But, even if a virus is weakened or killed, it still can sometimes cause side effects, and vaccines with weakened viruses still can be dangerous to people with compromised immune systems.

Studying virus-infected cells in Dr. Chen’s lab in the College of Pharmacy.
Scientists can either manufacture the protein that helps generate an immune response, or purify an existing protein from a virus for use in a vaccine. There are drawbacks, though. Many viruses have more than one protein that can generate an immune response. Therefore, it can be challenging to discover which combination will work best to stimulate the strongest immune defenses against a specific virus.
Utilizing our own body to create defenses: nucleic acid vaccines
Another, more advanced method of vaccine development uses the genetic material in DNA and RNA, called nucleic acids, to stimulate the body itself to manufacture the viral protein that triggers the immune system to recognize the pathogen. This is, so far, the fastest method to a vaccine, Dr. Chen said.
Researchers first sequence the DNA and RNA in a virus, then test which of those protein-coding sequences induce sufficient immunity. Next, they develop a vaccine that prompts the body to produce just those proteins and trigger the immune response to create antibodies to the virus.
COVID-19 vaccine development
Once a vaccine is developed using one of the above methods, human trials begin.
Dr. Chen said the current frontrunners in the COVID-19 vaccine development process all use the nucleic acid approach, which is why the development has been rapid. Chinese researchers shared the genetic sequence of the virus that causes COVID-19 earlier this year, enabling other scientists to figure out which viral proteins induce immunity.
A research scientist analyzes cellular proteins from samples of a viral infection
Dr. Chen said the big challenge for a pandemic vaccine comes in Phase III trials, when scientists determine whether recipients are actually protected from the virus. The best way to test this is in healthy volunteers who live in regions where the pandemic is active. Some receive the vaccine and some receive a placebo, and infection rates are tracked in both groups.
“You have to test it in an environment where there are active transmissions to see if it provides protection,” Dr. Chen said.
Production timeline
If a Phase III trial shows a COVID-19 vaccine is safe and effective, and it receives approval from the FDA, the next step is for a manufacturer to produce enough doses of the vaccine to make it widely available.
To avoid an ethical dilemma regarding who should receive a vaccine, Dr. Chen said it’s important to have at least hundreds of millions of the vaccine available for distribution.
In production, the biggest financial hurdle is building or refitting manufacturing facilities for the specific vaccine. Dr. Chen said this step is being hurried along with government funding and the Defense Production Act, which are enabling the federal government to expedite the manufacture of supplies and materials, including a vaccine, during an emergency.

