Dynamic Nature of Genome-Protecting DNA May Offer Clues to Fight Cancer
Researchers propose a new paradigm for analyzing genetic mutations after study shows varying levels of heterochromatin, a key player in genomic stability.
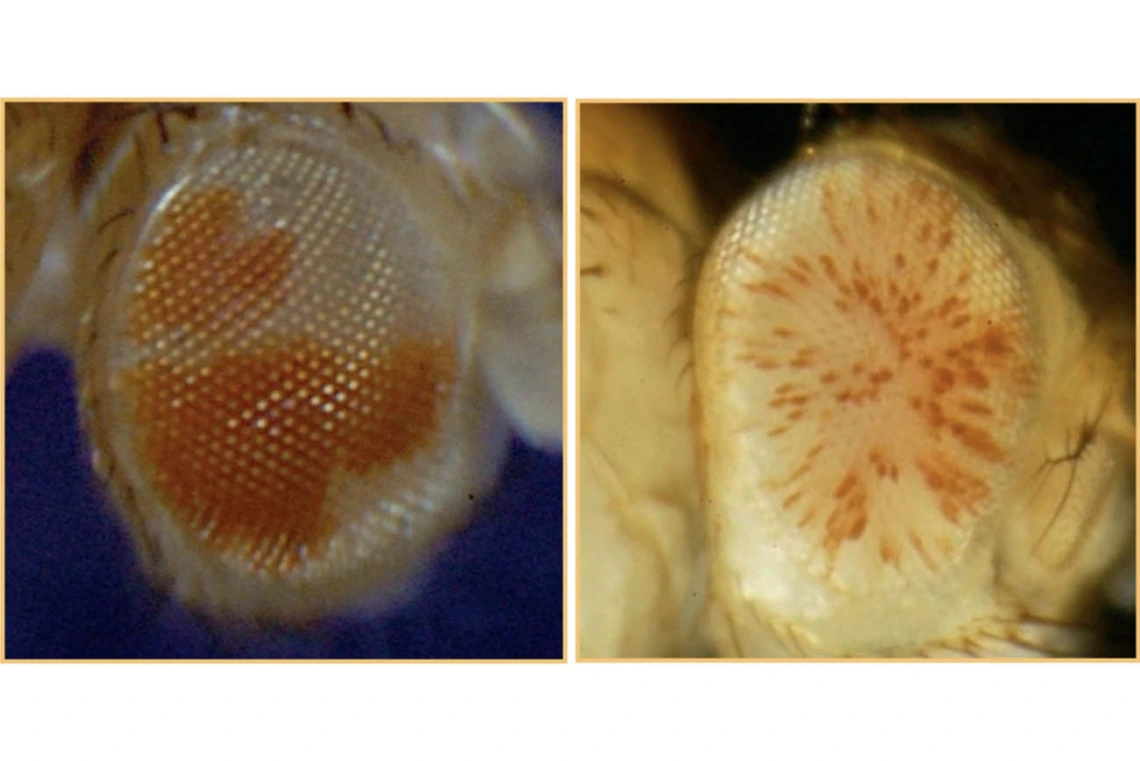
Researchers used the mutations that lead to random and compelling patterns of eye color in Drosophila to study heterochromatin, a form of DNA that plays a vital role in maintaining the stability of chromosomes by silencing transposable elements.
University of Arizona Cancer Center researchers uncovered a potential epigenetic basis for the genomic instability that is a hallmark of many types of cancer. The finding, published in the journal Proceedings of the National Academy of Sciences, paves the way for researchers to explore new ways to treat or prevent cancer.
The study found that levels of heterochromatin, a form of DNA that plays a vital role in maintaining the stability of chromosomes by stopping mutations, are dynamic and not stable, as previously thought.

Keith Maggert is a member of the UArizona Cancer Center and an associate professor in the UArizona College of Medicine – Tucson’s Department of Cellular and Molecular Medicine. (Credit: Kelvin Pond)
Transposable elements, or jumping genes, are sequences of DNA that can move from one location on the genome to another. They cause mutations by turning genes on or off, depending on where they land. Heterochromatin’s main function is to protect the genome by silencing transposable elements and preventing the instability of repeated sequences of DNA.
“Some of the defects we see early in cancer development are actually caused by these transposable elements moving around,” said Dr. Maggert, who conducted the research with graduate student Farah Bughio. “Up until now, heterochromatin was perceived as being stable and pretty good at its job – it would shut off the transposable elements and they would stay off. But we showed that heterochromatin is dynamic, cycling on and off through life.”
Building on prior research, the UArizona Health Sciences researchers used a switch monitoring system they developed to monitor mutations during position-effect variegation, a rearrangement of genes that results in random and compelling patterns of eye color in Drosophila.
Dr. Maggert and Bughio found that when heterochromatin levels were high, transposable elements were silent, but when heterochromatin weakened, transposable elements became more active.
“Our study proved that heterochromatin is very dynamic and that transposable elements are dangerous throughout our lives,” Dr. Maggert said. “We have to be very cognizant of what we do to our cells in order to keep heterochromatin safe. Unfortunately, a lot of the chemotherapeutics that are directed at gene expression have a consequence: they weaken heterochromatin. If heterochromatin is already struggling to do its job, then taking some chemotherapeutics may allow these transposable elements to move more frequently. That contributes to the problem rather than solving it.”
In a complementary line of research, Dr. Maggert is working on determining the environmental conditions that cause heterochromatin to weaken. He has published results showing that heterochromatin weakens as people get older and when they eat too much.
“We're trying to accumulate a list of things that cause heterochromatin to weaken, and we're trying to find ways to restrengthen it because we now know that heterochromatin is synonymous with genome stability for life,” he said.
This study was supported in part by the National Institutes of Health (R01GM123640, P30CA023074, P40OD018537).
Contact
Margarita Bauzá
313-520-2109
mbauza@arizona.edu

