Falloposcope cancer screening tool testing advances to next steps
Jennifer Barton, PhD, is spearheading an effort to diagnose ovarian cancer earlier and give high-risk patients a new option.
Jennifer Barton, PhD, has spent decades working to improve the timeline for detecting ovarian cancer. She and her collaborators are now conducting two pilot trials using optimized versions of her patented falloposcope – the first endoscope to image and collect cells from fallopian tubes.
The new in vivo and ex vivo studies are testing the device with 200 patients, adding to data from about 20 participants in previous investigations. The researchers are expanding the foundation they’ve steadily built with the help of emerging technologies. Barton and her collaborators plan to further refine the device and move it toward clinical adoption.

Jennifer Barton, PhD, serves as interim vice provost for health programs at the University of Arizona Health Sciences, overseeing research, administration and the clinical partnership with Banner Health.
Kris Hanning, U of A Office of Research and Partnerships
Helping women with cancer and those at high risk
The falloposcope journey began around 20 years ago with a heartfelt appeal to Barton, the Thomas R. Brown Distinguished Professor of Biomedical Engineering and the interim vice provost for Health Programs.
“A gynecological surgeon friend of mine ran up to me, grabbed me and said, ‘You have to work on ovarian cancer. You’re a tool builder. You’re a problem solver. Solve this problem for me.’ We’ve been working on it ever since,” she said.
Researchers had begun investigating how ovarian cancer starts. About 12 years ago, the community reached a consensus that most deadly forms of the disease start in the fallopian tubes. Physicians lacked a way to examine the fallopian tubes at high resolution – they often only identified the cancer when it was too late. Together, that information presented an opportunity to detect the disease at a precancerous or early stage, explained Barton, who is also a professor of optical sciences.
The five-year survival rate for American women diagnosed with ovarian cancer before it metastasizes is 92%, but these patients make up only about a quarter of cases, according to the National Cancer Institute. For those whose cancer has spread, the five-year survival rate is 32%.
An endoscope aids diagnosis efforts by providing real-time imaging of internal organs and cavities. Barton invented the falloposcope version to save lives through early detection and help patients who are at high risk avoid unnecessary removal of the ovaries and fallopian tubes.
Patients are identified as high-risk through genetic testing and family history analysis. One of the most common findings is a mutation in one of two genes – BRCA1 or BRCA2 – which prevents the genes from repairing damaged DNA. This allows DNA damage to build up, increasing the likelihood of cancer.
Currently, women who are at high risk for ovarian cancer have two choices, Barton said. They can undergo surgery at age 35, or they can opt for surveillance through blood tests and ultrasound. But research indicates those methods aren’t reliable for early detection, she added.
“What it comes down to is, you either get the surgery or you take your chances.”
The prophylactic surgery is highly effective at preventing cancer from developing, but it also brings undesired consequences that can include reduced life expectancy. It also eliminates the possibility of natural conception and abruptly introduces menopause. Also, many women who choose to undergo the procedure would never have had the disease, Barton said.
“For those women, even though their lifetime chance of getting ovarian cancer may only be 20%, the fact that ovarian cancer is almost always deadly means they are counseled to get surgery when they’re 35. And that’s just awfully young,” she said.
Taking on the challenge
No early detection method exists because there are inherent obstacles to reaching the target area with imaging and biopsy equipment.
“A key focus area is learning how to thread the endoscope through all of the twists and bends in the fallopian tube,” said John Heusinkveld, MD, Barton’s longtime clinical research partner and a Banner Health physician and surgeon.
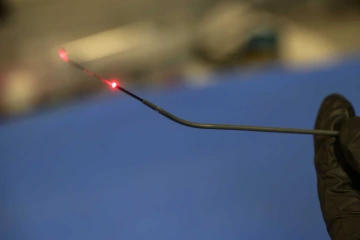
The falloposcope, a diagnostic device less than 1 millimeter in diameter, is engineered to gently move past fallopian tube cilia. Its red light provides laser illumination for viewing tissue, which can offer insights into tissue health.
Kris Hanning, U of A Office of Research and Partnerships
Not only are the tubes twisty and tiny, but they’re also filled with finger-like projections called cilia, which move eggs and sperm. The instrument must have a diameter of less than 1 millimeter and be slick enough to pass through without catching on cilia while also offering advanced imaging sensitivity.
“It’s a challenge, but it’s absolutely possible with modern technology,” Barton said. “We’ve been working on this for so long because we’ve had to wait for developing technology, including lasers, fibers and materials. Things that weren’t possible when I first looked into this are possible today.”
Barton secured initial funding from the U.S. Department of Defense Ovarian Cancer Research Program in 2018. Since then, she has raised additional support from the DOD, the National Cancer Institute and private donors. The team began the first human trial in 2021.
Barton and her team have published studies showing the device is safe and renders high-resolution structural and biochemical images. They’ve also documented the baseline for normal fallopian tube tissue and made improvements to the falloposcope based on the clinical experiences of Heusinkveld, also an associate professor of obstetrics and gynecology at the College of Medicine – Tucson. They incorporated the latest design in the new pilot trials.
One trial, conducted locally in partnership with Banner Health, is focused on 10 participants who are at normal risk for ovarian cancer and having surgery for reasons other than cancer. This effort will generate valuable insights about the device’s mechanics and imaging.
The other trial is underway with partners at New York-Presbyterian Queens Hospital, which runs a clinic for patients at high risk for ovarian cancer. Collaborators there are using the falloposcope to test tissue directly after its removal in surgery. The researchers will analyze the findings to test the falloposcope’s ability to distinguish the earliest signs of cancer.
The path to patients
Barton estimates the current trials will conclude in 2027, giving the researchers data from a combined total of 200 women. Next, she hopes to identify an industry partner to license the invention, conduct additional studies and obtain approval from the Food and Drug Administration. Barton is working with Tech Launch Arizona, the university’s commercialization arm.

Dilara Long, an MD/PhD candidate at the U of A College of Medicine – Tucson, assists with the diagnostic use of the falloposcope and is exploring additional applications for the device.
Kris Hanning, U of A Office of Research and Partnerships
Barton and Heusinkveld envision falloposcope testing as an outpatient procedure. Not all women would need the test routinely, though Barton would like to see the technology made available to those at very high risk first.
“I lost a good friend to ovarian cancer at age 35. Her mother died young of ovarian cancer, too,” Barton said of a friend who has remained on her mind as she develops the falloposcope. “I’m quite sure she had a genetic abnormality, and she could have been somebody that maybe we could have helped.”
Barton believes the falloposcope can be developed to serve additional patients and purposes beyond cancer.
“If we’re imaging the fallopian tubes and collecting cells, we should be able to look not just for cancer, but for other diseases,” she said.
Barton and her collaborators are beginning investigations into using the falloposcope to determine causes of infertility. She believes it could also allow physicians to test cancer prevention treatments by examining the reactions of cells.
“I’m proud to work on those new devices and techniques that will solve problems,” Barton said, “and I’m really excited to continue working with my physician collaborators.”
Experts
Jennifer Barton, PhD
Interim Vice Provost for Health Programs
Thomas R. Brown Distinguished Chair, Department of Biomedical Engineering, U of A College of Engineering
Professor, Biomedical Engineering, U of A College of Engineering
Professor, Electrical and Computer Engineering; U of A College of Engineering
Professor, Optical Sciences, U of A College of Optical Sciences
Professor, Agricultural-Biosystems Engineering, U of A College of Agriculture, Life & Environmental Sciences
Member, U of A Comprehensive Cancer Center
Member, BIO5 Institute
Contact
Blair Willis
U of A Health Sciences Office of Communications
520-419-2979, bmw23@arizona.edu

