Miniature organs driving precision medicine, drug discovery
University of Arizona Health Sciences researchers are using organoids to design novel therapies and better understand cancer and rare diseases.

Organoids are miniature tissues or tumors grown in the laboratory that provide scientists with a more realistic model to design therapeutics for hard-to-treat cancers and rare diseases.
President Richard Nixon declared war on cancer in 1971 with the signing of the National Cancer Act. Despite more than a half-century of scientific breakthroughs, cancer remains the second-leading cause of death in the United States.

Yana Zavros, PhD, said that interest for using organoids in research is growing. She hopes to introduce more investigators to this approach and technology.
The challenge for scientists is that cancer is not universal. There are more than 100 types of cancer, and each of these cancers can have various subtypes. Even the same cancer type is not the same in any two patients, making the war on cancer a truly personal battle.
Now, researchers at the University of Arizona Health Sciences are turning to a new tool in the fight against cancer and other rare diseases: organoids.
“Organoids are tissues or miniature tumors grown in the laboratory,” said Yana Zavros, PhD, professor and associate head for research in the UArizona College of Medicine – Tucson’s Department of Cellular and Molecular Medicine and UArizona Cancer Center member.
Organoids are three-dimensional structures that mimic the genetic and biological functions of organs and provide researchers with an under-the-microscope look at how tumors may behave in the body. With this information, investigators can create tailored therapies for more effective responses in patients.
Dr. Zavros leads the Biology Development and Research of Organoids, or BioDRoid, service at the UArizona Cancer Center. BioDRoid collects and catalogs healthy cells, tumor cells and immune cells for many conditions and provides the expertise required to grow organoids from stem cells or patient tissue samples. The organoids can then be directly studied, tested with drugs or be cryopreserved for future investigation.
“The complexity of the organoids we grow in the lab closely mimics the patient’s own tumor behavior and how it responds to drugs in the body,” Dr. Zavros said. “Organoids allow us to look at the tumor microenvironment at a higher resolution. This helps us identify targeted therapies that can be tested with drugs to determine the safest and most effective treatment prior to clinical use in the patient.”
One small sample, one giant leap for research
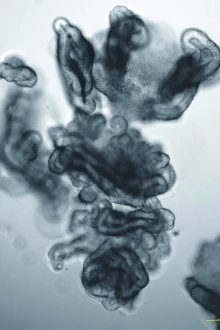
Patient-derived colonic epithelial organoid self-organize into a mini gut-like structure when cultured in a petri dish.
Once a tissue sample is received, BioDRoid processes the tissue and places it in an incubator to allow the organoid cells to begin growing. For aggressive tumors, organoids will develop within 24-48 hours. Within a week, hundreds of organoids will have grown. Researchers can continue expanding the organoids into the thousands until they have enough for a study. The ability to cryopreserve live organoids makes them a limitless resource.
Organoids offer several advantages to other common research methods, one of which is less reliance on animal models as the researchers can work with live human tissue. Organoid modeling is faster and easier than animal modeling and maintains a more realistic human physiology.
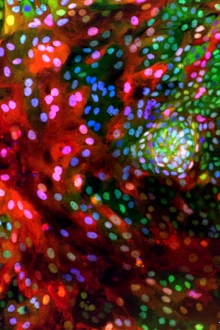
The cell nucleus (blue) and communication pathways (red and green) are identifiable in a genetically engineered organoid.
“Organoids give us a better representation of the cellular diversity of a tumor,” Dr. Zavros said. “Cells lines come from one patient and are not representative of the entire cell population. We can’t develop effective therapies based on five cell lines because those five patients may not be representative of the full population of patients that have a disease.”
Instead, organoids are valuable to researchers because they can be grown to mimic all the various subtypes of a specific disease. This leads to delivering more precise treatments to more people.
“Precision medicine to us is not collecting a tissue sample from every single person. That is not feasible,” Dr. Zavros said. “But what we can do is stratify and subtype organoids similar to how there are subtypes of cancers. This is important because we know that these subtypes of cancers are more responsive to certain therapies. We hope that the end result is that we can discover therapies that will help patients.”
One example of this translational research is a pancreatic cancer clinical trial led by Rachna Shroff, MD, MS, professor and associate dean of clinical and translational research for the College of Medicine – Tucson and medical director of the clinical trials office at the UArizona Cancer Center. Dr. Shroff is studying a new drug combination for pancreatic cancer patients whose tumors have been resistant to standard therapies.
Organoids are tissues or miniature tumors grown in the laboratory.
In the study, Dr. Shroff and her research team collect patient tumor biopsies prior to and during treatment. In collaboration with Dr. Zavros’ laboratory, the biopsies are used to grow organoid cultures to better understand the resistant cell populations within the tumor. The findings could eventually lead to new standard treatments for pancreatic cancer patients.
Providing a better platform
One of the limiting factors in drug discovery trials is the balance between a drug being effective at fighting off harmful cells while not destroying healthy cells. This balance becomes critical in the gut, where the lining of the stomach, intestine and colon absorb nutrients while eliminating harmful bacteria. However, this lining is constantly replacing old cells and regenerating itself, making it susceptible to disease if it regenerates abnormally.

(From left) Curtis Thorne, PhD, and Kelvin Pond, PhD, examine the cell structures of an organoid.
Curtis Thorne, PhD, assistant professor of cellular and molecular medicine in the College of Medicine – Tucson and UArizona Cancer Center member, leads a research laboratory focused on understanding how the lining of the gut maintains itself. His lab has been using organoids for the past six years to gain a clearer picture of what is taking place in the gut and how drugs can be developed to treat diseases like colon cancer.
“My lab views organoids as a drug-discovery platform,” Dr. Thorne said. “The way to think of them is as mini guts in tiny wells. We are able to put these miniature guts into 96- or 384-well plates. That allows us to do lots of experimental conditions to figure out how they grow and to discover new therapeutics that would prevent tumor organoids from growing.”
Dr. Thorne explains that drug toxicity is of particular concern for patients undergoing treatment and barrier function problems, such as those in the lining of the gut, can be deadly. By using organoid models, Dr. Thorne’s research team can better predict which drugs are the safest for further investigation.
“We are seeing that we can predict drug toxicity at a much earlier stage of the drug development. Generally toxic compounds can be triggered early, and we can focus our efforts on those that are expected to have better toxicity profiles when tested in patients,” Dr. Thorne said. “We think organoids are the best platform for demonstrating tumor behaviors in a dish in a form that we can screen drugs and identify better lead compounds that are more likely to work once they hit the clinic.”
Our Experts
Yana Zavros, PhD
Professor and associate head for research, Department of Cellular and Molecular Medicine, College of Medicine – Tucson
Shared Resource Director, Tissue Acquisition Cellular and Molecular Analysis, UArizona Cancer Center
Curtis Thorne, PhD
Assistant professor, Cellular and Molecular Medicine, College of Medicine – Tucson
Member, BIO5 Institute
Member, UArizona Cancer Center
Contact
Blair Willis
Health Sciences Office of Communications
520-419-2979
bmw23@arizona.edu

