Rebuilding nature’s blueprint to target cancer
A postdoctoral researcher is exploring how cantharidin, a natural toxin blister beetles use as a defense mechanism, could lead to safer and more effective cancer therapeutics.
Kevin Andre Scott, PhD, likens his research to building Legos on a molecular level.

Kevin Andre Scott, PhD, purifies a red mixture containing a cantharidin analog to isolate a pure compound, essential for testing its effectiveness against cancer.
Photo by Kris Hanning, U of A Office of Research and Partnerships
“Nature provides the basic building blocks,” said Scott, who is a postdoctoral fellow in the University of Arizona R. Ken Coit College of Pharmacy. “We’re just rearranging them to create something new.”
In Scott’s case, the building blocks make up a molecule produced by a notorious insect: the blister beetle, named for the blisters caused by a noxious liquid it secretes from a special gland. The blisters are a result of contact with cantharidin, a potent, natural toxin used medicinally for more than 2,000 years, with mentions in ancient Chinese pharmacopoeias.
Scott is working to unlock its potential to fight cancer. He began investigating cantharidin as a doctoral student.
“We made many molecules, but I only published one of the cantharidin derivatives in my dissertation,” said Scott, who did his doctoral work under Jon Njardarson, PhD, a professor of chemistry and biochemistry in the U of A College of Science and BIO5 Institute member. “Without funding to take the research forward, the rest were left on the shelf.”
That changed last year. Scott joined the lab of Wei Wang, PhD, a professor of pharmacology and toxicology and U of A Cancer Center member who plays a key role in advancing drug discovery and development while enhancing translational research collaboration at the university. Soon after, Scott was awarded a postdoctoral fellowship from the U of A Health Sciences One Health initiative.
“I came back to this project that previously would have just been left unfinished,” Scott said. “Cantharidin is probably the most interesting molecule you’ve never heard about.”
The blister beetle’s toxic gift
More than 1,500 species of blister beetles use cantharidin as both a chemical defense and a romantic gesture. In nature, males offer the compound to females during mating, who in turn use it to protect their eggs. If a predator or an unsuspecting person encounters the beetle, the compound can cause blisters by killing skin cells.
Scott, a self-described amateur entomologist, was initially drawn to the cantharidin out of personal fascination with insects and their chemical defenses. That curiosity soon intersected with a scientific problem: despite its therapeutic promise, cantharidin is rather difficult to work with in the lab.
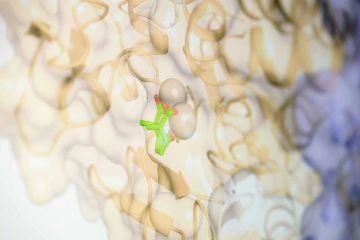
Scott creates molecules to attach to unique locations on an enzyme using a feature that allows swappable parts, like snap-on pieces on a toy.
Photo by Kris Hanning, U of A Office of Research and Partnerships
Building on his graduate and postdoctoral research, Scott discovered a new way to create molecules like cantharidin using a molecular feature that allows swappable parts, like snap-on pieces on a toy. By swapping out chains of atoms, his lab can tweak the molecules to make them safer and more effective, opening new possibilities for cancer treatments that were previously out of reach.
The innovation has yielded dozens of new analogs with a specific carbon-chain feature – more than double the number synthesized in cantharidin’s 100-year research history – and vastly expanded the chemical possibilities for drug development.
“This molecule helps kill cancer cells by blocking proteins called phosphatases, which some cancers can harness in mechanisms used to survive treatments,” Scott said. “The potential in our new technology lies in making the molecule more specific, so it targets only the right proteins, possibly becoming a powerful tool for cancer therapy.”
Cantharidin is small and looks deceptively simple but rebuilding the natural version of the molecule from scratch requires extraordinary precision.
"It’s like recreating a Lego sculpture but you don’t know which bricks you need or how to connect them,” Scott said. “If one brick, like a methyl group, is misplaced, the molecule won’t work or could become too toxic.’"
Scott says that is the challenge of cantharidin: its methyl groups make it both more potent and more toxic.
“We need to thread the needle between potency and toxicity,” Scott said. “By removing one methyl group and adding customizable side chains, we’re making safer, more effective versions.”
Designing a smarter cancer drug
Scott’s research focuses on refining cantharidin analogs to inhibit phosphatases, which are enzymes that regulate practically every vital process in every cell in your body. When these enzymes malfunction or become overactive, diseases such as cancer can develop.
“Most enzyme inhibitors fail to fully inhibit the enzyme, or they hit too many of them at once,” Scott said. “That’s when you get serious side effects. There are very few Food and Drug Administration-approved phosphatase inhibitors. In developing promising new phosphatase-targeting drugs, what we’re missing are molecules that are both potent and selective.”
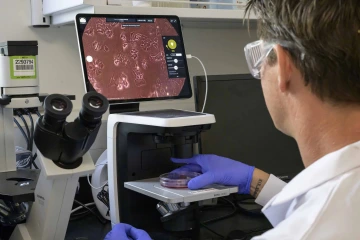
Scott examines cancer cells growing into clusters on a plate, where he cultivates them and tests how his cantharidin analogs disrupt their growth.
Photo by Kris Hanning, U of A Office of Research and Partnerships
Selectivity is especially tricky. The place where proteins bind on phosphatases looks nearly identical across different types of phosphatase within each family of the enzyme. As a result, a drug meant to block one enzyme often affects others, too, causing unwanted side effects.
Scott’s lab is working around this by designing molecules that attach to parts of the enzyme that are more unique. His team is also studying other compounds that may disrupt the growth or spread of cancer cells.
“We want to kill the cancer cells without killing healthy cells,” he said.
Scott’s research is in the preclinical stage, but the early findings are encouraging. His lab tested the new molecules on human cancer cells, including breast and colorectal cancer, and observed selective activity with minimal harm to healthy cells.
The next phase involves moving into animal studies. His lab is preparing to test the compounds in mouse models to better understand their safety and effectiveness in a living system.
“This is a critical step,” Scott said. “It’s how we bridge the gap between chemistry and clinical relevance.”
As new data emerges, Scott’s work could help launch a new line of cancer therapeutics designed around molecules that use nature’s blueprint as the foundation for lifesaving innovation.
“This research brings together everything I’m passionate about: chemistry, biology, medicine and even my love of insects,” he said. “We’re learning from nature, adapting its lessons, and hopefully creating something that can make a real difference.”
Scott is not only developing a better cancer drug but also showing how natural products, reimagined through modern science, can yield powerful treatments for devastating diseases.
Experts
Kevin Andre Scott, PhD
Postdoctoral Fellow, R. Ken Coit College of Pharmacy
Related Stories
Contact
Blair Willis
U of A Health Sciences Office of Communications
520-419-2979, bmw23@arizona.edu

