An Essential Bridge Between Patients and Research
A passion for people and keen scientific knowledge drive Sylvia Paton to deliver compassionate care as a research nurse at the UArizona Cancer Center.

A passion for people and keen scientific knowledge drive Sylvia Paton to deliver compassionate care as a research nurse at the UArizona Cancer Center.
Clinical trials can provide hope to cancer patients facing even the most devasting of diagnoses. While these studies are promising, many patients become overwhelmed and frightened on an uncertain journey in their battle with cancer.
“These patients need someone to help guide them through the maze of being in a clinical trial,” said Sylvia Paton, MSRN, a senior research nurse at the University of Arizona Cancer Center. “Their journey could last only a couple months if the treatment fails. It could last much longer if successful, and that’s what we all want. But a research nurse is always that link alongside them through their journey.”
A passion for people
Born in Mexico, Paton moved a lot while growing up. By age nine, she also had lived in the United States and Canada. She returned to Mexico to pursue a degree in biochemistry from Tecnológico de Monterrey and later received her master’s in education from Framingham State University in Massachusetts.

Sylvia Paton, MSRN, spent 15 years as a middle school science teacher before transitioning into a career as a nurse.
“I’ve always liked people and I like challenges” Paton said. “As a teacher, I built relationships with hundreds of middle school students and parents. I started to think about nursing because nursing is all about people. For me, it was the perfect combination of people and science.”
In 2015, Paton completed the Master of Science for Entry into the Profession of Nursing degree program at the UArizona College of Nursing. Following two years as a bedside nurse at Banner – University Medical Center Tucson, she was ready for yet another challenge, one that would allow her to combine the nursing skills she acquired with cutting-edge scientific exploration.
A very odd job
While considering potential next steps in her career, Paton came across an unfamiliar description for a position at the UArizona Cancer Center: research nurse.
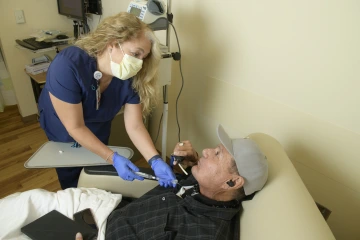
As a senior research nurse at the UArizona Cancer Center, Paton will regularly see as many as 18 patients across more than a dozen clinical trials.
When Paton started as a research nurse in January 2018 under Julie E. Bauman, MD, deputy director at the Cancer Center, she was one of only four research nurses on staff. The team now has 11 research nurses who are assigned to various clinical research teams.
The responsibilities of a research nurse are similar to those of other nurses but with added complexities working with clinical trial patients. Among those are the protocols written specifically for every clinical trial, including careful attention to the dosage and the potential side effects of the medication. This attention to detail is of critical importance during Phase I trials, for example, which are studying new treatments in humans for the first time.
“I'm kind of like a safety measure to make sure that we are following the protocol,” Paton said. “I am that last check before a patient gets their medication, so that's a part of my job that I take very seriously to ensure patients get the right medication, the right dose, with the right timing.”
“For me, it was the perfect combination of people and science.”Sylvia Paton, MSRN
Additionally, a research nurse is essential in the data collection and reporting to the sponsors of a clinical trial. Any symptoms that patients experience while on a trial are collected and reported to all parties, including the U.S. Food and Drug Administration, the drug manufacturers and any other third parties sponsoring the study. This data helps determine whether an experimental treatment moves forward and receives approval for more widespread use.
“The meat of the data we collect are the adverse events,” Paton said. “We all see the television commercials for drugs where at the end they read a long disclaimer listing a bunch of possible symptoms and side effects. Well, all of that is collected by us as research nurses during these studies.”
Dr. Bauman considers Paton the perfect example of what a research nurse should be.
“She's the full package,” Dr. Bauman said. “She is very intelligent, and she has amazing interpersonal skills. But she also has a remarkable ability to understand the science and apply it to the patient's experience, and on top of that has wonderful relationships with her patients.”
Bridge between patient and investigator
Before a clinical trial begins enrolling patients, there is an extensive amount of preparation required of all parties. Pre-clinical data must first demonstrate safety and effectiveness before regulatory entities approve a new trial. At the Cancer Center, this includes the Scientific Review Committee, which oversees and ensures the scientific merit, priority, value and progress of all cancer-related clinical studies.

Darrell Dean Rice (left) has formed an everlasting bond with Paton over the course of four clinical trials since his original diagnosis with cancer of the larynx in 2017.
Once a trial is ready to launch, the study’s principal investigator will share the protocols with a clinical research team, which includes a research nurse. They are provided with a long document – sometimes more than 200 pages – that outlines the protocols for the trial. The protocols include the overview of the study detailing why researchers think the new treatment will work and who should be included in the study. It also provides pharmacological information and other scientific background that the research nurses need to know.
“My goal is to understand the treatments and wrap my head around what it’s going to look like in the clinic,” Paton explained. “What does it mean for me as a nurse? Do we have the capabilities? And then I think about what the experience will be like for the patient. How many hours will the patient be here? How often do we need to bring them in for these treatments? There is a lot to consider before we ever see a patient.”
While clinical trials take on a broader goal of improving care and treatment for all patients, it is the patients in the trials who take precedence for Paton and her fellow research nurses.
“They are the glue for putting together the patient's experience, but also that continuous source of monitoring to see how they respond to the treatment,” Dr. Bauman said. “We know that patients who participate in clinical trials have better outcomes not necessarily just because of the treatment they're receiving from the drug itself, but from the team of people that provides this additional level of coordination oversight to their care.”
“She’s like family”
Darrell Dean Rice is one of Paton’s patients. He was diagnosed with cancer of the larynx in 2017. Initial treatments were unsuccessful, and Rice’s disease eventually spread into an uncontrollable head and neck cancer. That’s when he met with Dr. Bauman and was introduced to Paton as his primary nurse.

Rice’s wife, Mary (right), says that Paton has helped pull Rice through some of his darkest times and reassured him that she is there to do what is best for him.
Rice’s journey has been difficult. Radiation has impaired his speaking ability to the point that he relies on an electrolarynx – a battery operated machine that produces sound to create a voice – to verbally communicate. He has participated in four clinical trials in his battle to beat the cancer, but none have been successful.
“There have been many times that I was ready to give up,” Rice admitted. “But Sylvia is always there for me, to encourage and support me. She is amazing. She’s like family.”
Rice remains hopeful that yet another clinical trial may be in his future to help cure his disease, but he is not currently enrolled in a study.
Still, Paton is a comforting influence on Rice and his wife, Mary.
“Sylvia tells me, ‘You have my phone number, you have my email,’” Mary said. “’Whatever Darrell needs, you let me know. We can get him in, don’t worry. I am always here for you guys.’”

