Reimagining autoimmune treatments with 5MCAR T cells inspired by nature
Researchers at the U of A Health Sciences Center for Advanced Molecular and Immunological Therapies are investigating the potential of genetically engineered T cells to treat autoimmune diseases.
(From left) Research scientist Mark Lee has worked for professor and immunologist Michael Kuhns, PhD, for years. Lee is now opening his own lab at the Center for Advanced Molecular and Immunological Therapies in Phoenix.
Photo by Noelle Haro-Gomez, U of A Health Sciences Office of Communications

Mark Lee received his bachelor’s degree from the University of Arizona in 2011 before joining the Kuhns Lab. He is now a principal scientist at the Center for Advanced Molecular and Immunological Therapies.
Photo by Noelle Haro-Gomez, U of A Health Sciences Office of Communications
Humans have a long history of borrowing from nature to advance technology. It is a concept that researchers including Mark Lee and Michael Kuhns, PhD, at the University of Arizona Health Sciences employ to study the natural mechanisms behind how immune cells function to generate immunological therapies.
“Our understanding of biology and the internal mechanisms that sustain life is ever increasing,” Lee said. “The idea that we can mimic some of what we know about nature to create new technologies and treatments is amazing to me.”
Lee, a principal scientist at the U of A Health Sciences Center for Advanced Molecular and Immunological Therapies, envisions a future where the body’s immune system can be harnessed to cure a variety of diseases. Through the work of Lee and others, CAMI is being developed to be a national biomedical research hub on the Phoenix Bioscience Core. The goal: advance precision medicine treatments for conditions including cancer, infectious diseases and autoimmune disorders.
Building a new model
For more than a decade, Lee worked alongside Kuhns, a professor in the U of A College of Medicine – Tucson’s Department of Immunobiology, to improve CAR T cell therapy, an immunotherapy used to treat certain types of cancer.
CAR T cells are manufactured by collecting a patient’s T cells and engineering them to produce chimeric antigen receptors, or CARs, that are designed to bind to certain proteins on cancer cells. The CAR T cells are transfused back into the patient, where they can go to work killing cancer cells.
“CAR T cell therapy has been a breakthrough, but it can sometimes be ineffective and also runs the risk of a severe inflammatory response,” said Kuhns, who recently was named CAMI’s senior scientific advisor. “Since the fundamental design of CARs is based on an early-1990s understanding of T cell biology, we think there is room for improvement. We’ve learned a lot more about these molecular machines since then.”
Today, scientists have a much more detailed understanding of what drives T cell activation. In nature, each T cell contains a receptor complex made up of five components: the antigen receptor module, three signaling modules, and a coreceptor module. These components convert information into instructions for the T cell to follow.
“Now that we’ve zeroed in on the building blocks that make up each module and the intricacies of how information is relayed through receptors and into the cell, there are even more opportunities to explore,” Lee said.
Designing new immunotherapy treatments

Michael Kuhns, PhD, focuses his research on how T cells are activated and carry out specific functions, and ways of directing these processes to improve vaccine or tumor responses, prevent transplant rejection or reduce autoimmunity.
Photo by Noelle Haro-Gomez, U of A Health Sciences Office of Communications
Several CAR T cell therapies are approved by the Food and Drug Administration to treat blood cancers, including lymphomas, some forms of leukemia and multiple myeloma. Using nature as a blueprint, Kuhns engineered a biomimetic receptor called a five-module chimeric antigen receptor, or 5MCAR, that can be added to T cells,
Kuhns and Lee are testing their engineered 5MCAR T cells against Type 1 diabetes, an autoimmune disease. In 2020, they co-authored a paper that described the 5MCAR T cell and its effectiveness against Type 1 diabetes in a nonobese diabetic mouse model.
“Type 1 diabetes develops when overreactive immune cells infiltrate areas of the pancreas and kill beta cells. Beta cells are responsible for producing the blood sugar-regulating hormone insulin,” Kuhns said. “Our paper showed evidence that we can direct different immune cells to kill the overreactive immune cells. This stops the process before it really gets going.”
They are encouraged by their findings. Lee’s initial experiments at CAMI will test how updated versions of 5MCAR T cells might be able eliminate Type 1 diabetes after it has already developed.
Lee and Kuhns are also collaborating on future generations of 5MCAR T cells they hope to advance to clinical trials. The ultimate goal, Lee says, is to develop a product to prevent and treat Type 1 diabetes in humans.
“Engineering is an iterative process: you build, test, refine and repeat,” Kuhns said. “That’s why modern cars don’t look anything like the Model A Fords built in 1903. We’ll be doing the refining in my lab and then taking that work to CAMI, almost like a showroom floor.”
Meanwhile, back in Tucson, Kuhns will be working on ways the 5MCAR can be fine-tuned to target different autoimmune diseases.
Back in the workshop
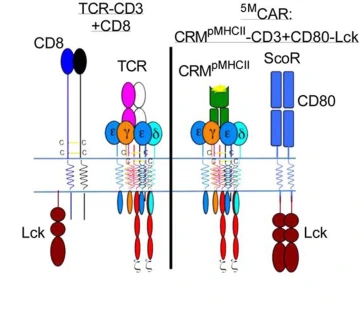
The illustration on the left represents a natural T cell, which was the inspiration behind the genetically engineered 5MCAR T cell, represented by the illustration on the right.
Illustration courtesy of Michael Kuhns, PhD
In addition to 5MCAR T cells, Kuhns and Lee are investigating CD4, a coreceptor that they found plays a more active role in regulating T cell receptor signaling than previously thought.
In a study published in eLife in July 2022, they examined the relationship between CD4 and T cell function through years of evolution in fish, reptiles, marsupials and mammals. They discovered sequences of amino acids, called motifs, on different parts of CD4 that seemed to strengthen or weaken its signaling power.
“The CD4 molecule has been evolving these motifs for millions of years, so we knew they must be important,” said Lee, who published a second paper about CD4 motifs in eLife in April. “This knowledge is likely to fuel future innovations.”
Lee and Kuhns are also investigating a novel way to redirect immune cells to fight cancer by capitalizing on their affinity for fighting viruses.
“T cells are very good at attacking and killing the viruses that they already recognize. We all have immune cells that are primed to fight viruses that we’ve either experienced or been vaccinated against, things like the flu or chicken pox,” Kuhns said. “To trick the T cells, we are going to show them a viral antigen they’ve seen before in an attempt to get them to kill the cancer tumors while trying to get to the virus they believe is inside.”
CAMI’s research projects are starting now in the Biosciences Partnership Building, where Lee – and sometimes Kuhns when he visits from his lab in Tucson – will be hard at work developing molecular machines that could revolutionize treatments for autoimmune diseases.
Experts
Michael S Kuhns, PhD
Professor, Department of Immunobiology, College of Medicine – Tucson
Senior Scientific Advisor, Center for Advanced Molecular and Immunological Therapies, U of A Health Sciences
Member, BIO5 Institute
Mark Lee
Principal Scientist, Center for Advanced Molecular and Immunological Therapies, U of A Health Sciences
Contact
Brian Brennan
U of A Health Sciences Office of Communications
520-621-3510, brianbrennan@arizona.edu

