Brain’s self-cleaning machinery may be key to Alzheimer’s
Specialized cells take out the trash in the brain. When they break down, waste builds up, possibly setting the stage for Alzheimer’s disease.
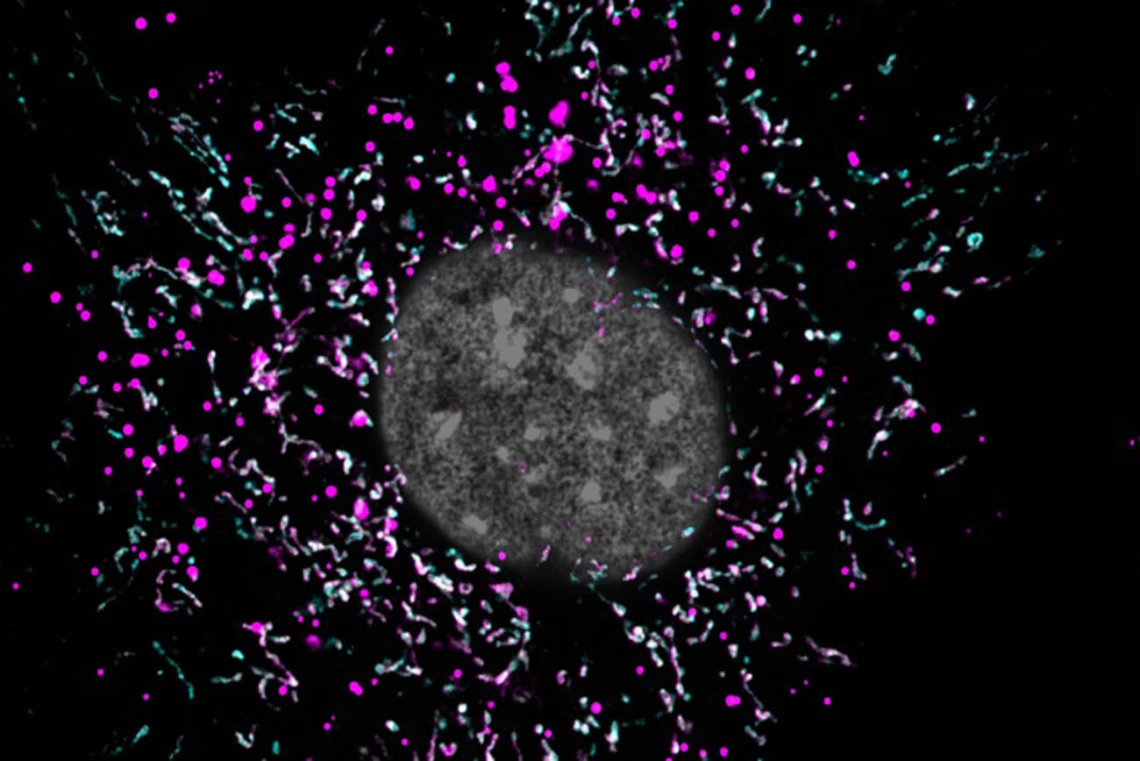
When an astrocyte’s mitochondria (magenta) are impaired, they are unable to break down lipid waste (cyan), and the buildup of extra fat (white spots) might be accelerating the progression of Alzheimer’s disease.
When he was growing up in China, Fei Yin, PhD, wanted to be a scientist.

Fei Yin, PhD, joined the Center for Innovation in Brian Science in 2017 as the assistant director of translational neuroscience.
“In elementary school, most children wanted to be scientists, but we didn’t really know what that meant and what it took,” he recalled. “Before I was applying for college, I didn’t know what scientific path to take.”
After completing his undergraduate studies in biochemistry, Yin matriculated at the University of Southern California, where his path became clearer. His doctoral adviser, Enrique Cadenas, MD, PhD, was an expert in mitochondria, the “powerhouses” of the cell that generate the energy it needs to do its job.
As a postdoctoral researcher at USC, Yin worked with Roberta Diaz Brinton, PhD, a world-renowned expert in Alzheimer’s disease. At that point, he knew he would devote his career to solving the mysteries of the mitochondria, untangling how they may be implicated in the progression of Alzheimer’s disease.
Expanding the search for Alzheimer’s origins
After becoming the inaugural director of the Center for Innovation in Brain Science at the University of Arizona Health Sciences, Brinton recruited Yin to join her in 2017 as the assistant director of translational neuroscience. Yin, currently an associate professor of pharmacology in the College of Medicine – Tucson, is still early in his career, but he has already made discoveries about how faulty mitochondria might lead to brain diseases such as Alzheimer’s.
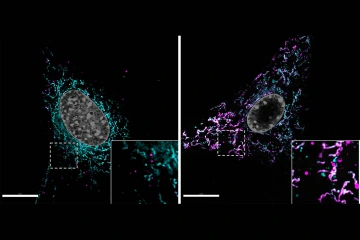
Lipid accumulation in a normal astrocyte (left) is compared with an astrocyte with dysfunctional mitochondria (right). The mitochondrial network is labeled in magenta and lipids accumulated within cells are labeled in cyan. White spots mark undegraded lipids trapped at the mitochondria.
Most mitochondria research in the context of Alzheimer’s focuses on neurons, brain cells that consume a lot of energy to keep us perceiving, thinking and forming memories. But the Yin Lab shifted its focus to astrocytes, star-shaped cells that support neurons and remove waste products from the brain — waste products including lipids, fatty compounds that are essential to brain function.
Lipids have been on the radar since 1907, when Alois Alzheimer, MD, noted abnormal lipid structures in autopsied brains. But the bulk of Alzheimer’s research focused on other phenomena, such as the buildup of abnormal protein structures including plaques and tangles in Alzheimer’s brains. Drugs targeting these proteins have yet to result in a cure, though, leading to an expanded search for Alzheimer’s root causes. Some scientists, like Yin, turned the focus to lipids in Alzheimer’s brains.
“About half the brain’s dry weight are lipids, but they have turnover,” Yin said. “Our studies suggest that mitochondria in astrocytes get rid of lipid waste in the brain.”
"“We believe that altered lipid turnover significantly contributes to neurodegenerative diseases, just as it does to metabolic and cardiovascular diseases."
Fei Yin, PhD
When an astrocyte’s mitochondria are impaired, they are unable to break down lipid waste, and the buildup of extra fat might be gumming up the works. The Yin Lab discovered that when astrocytes are unable to efficiently process this waste, lipids accumulate in the brain like garbage piling up on the sidewalk, harming their neighboring cells and the brain environment.
Yin is now testing the hypothesis that lipid accumulation initiates or accelerates the progression of Alzheimer’s disease.
“Lipid balance is important to maintain for brain function. If the balance is disrupted, there will be consequences,” he said. “We believe that altered lipid turnover significantly contributes to neurodegenerative diseases, just as it does to metabolic and cardiovascular diseases.”
Moving toward drug discovery
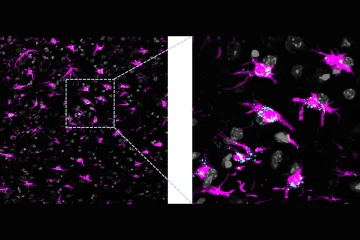
Lipids accumulation in a brain with dysfunctional astrocytic mitochondria. Astrocytes are labeled in magenta and lipids accumulated within cells are labeled in cyan.
Human DNA might also hold clues about why some mitochondria go awry. The APOE4 gene is the greatest genetic risk factor for Alzheimer’s, which was previously shown to be associated with plaques and tangles. When Yin looked at the gene through his lipid-focused lens, he was intrigued to find that it was also implicated in mitochondrial dysfunction and lipid imbalance in astrocytes.
One of the most important tools an astrocyte has for cleaning up lipids is a protein called ApoE, which grabs hold of lipids to carry them around. Some people have a misshapen form of this protein, called ApoE4, that can’t grasp lipids as firmly.
“We found that compared to the normal form, ApoE4 has a reduced ability to transport lipids to mitochondria in astrocytes for degradation,” Yin said. “Now we’re trying to determine how this disrupted lipid metabolism in early stages is connected to key hallmarks of the disease: plaques, tangles, neuronal loss and cognitive impairment.”
Yin says his discoveries about lipid dysregulation as a central mechanism driving Alzheimer’s disease – and ApoE4’s role in this dysfunction – is a highlight of his career so far.
“It was so exciting when we found this,” he said. “I hope our work and other groups’ work can find a therapeutic that targets lipid metabolism and can be used for Alzheimer’s disease.”
The Yin Lab is currently testing new therapeutics that they hope will boost astrocytes’ ability to clear excess lipids and prevent cognitive decline in Alzheimer’s animal models. Yin hopes his discoveries will apply to other neurodegenerative diseases as well.
“Disrupted lipid metabolism is not unique to Alzheimer’s disease. There is accumulating evidence demonstrating lipid dysregulation in Parkinson’s disease, in ALS [amyotrophic lateral sclerosis], in Huntington’s disease — nearly all neurodegenerative diseases,” he said. “It could be a common mechanism underlying these devastating diseases.”
A lifelong love of science
Thinking back to his childhood, when he knew he wanted to be a scientist but wasn’t sure what that would look like, Yin says he is happy with where his journey has taken him.

Bioenergetic dysregulation often occurs in parallel with widespread neuroinflammation, Yin says, yet how these processes interact is unclear. His research focuses on delineating the crosstalk between brain energy metabolism and chronic neuroinflammation, specifically cellular redox dysregulation and lipid dyshomeostasis as candidate-bridging factors and potential therapeutic targets for age-associated neurodegenerative disorders.
“I don’t know if I anticipated it to be like this, but choosing to study mitochondria in the brain was the right decision,” he said.
Yin says he always has dozens of scientific journal articles open on his computer at any given time, and with so many ideas out there, he will never run out of riddles to solve and problems to tackle.
“The scientific community still does not know exactly how the brain becomes malfunctional in neurodegenerative diseases. The brain is so complex — so many different kinds of cells, all these connections between different cells, across regions, and even crosstalk outside the brain to the peripheral organs. Because of that, neurodegenerative diseases are difficult to treat,” he said. “This area has a lot of potential and opportunities for me to explore.”
Yin says the Center for Innovation in Brain Science gives him the platform and resources that enable these explorations. He looks forward to the day when his lab’s hard work results in an effective Alzheimer’s therapy that can be shared with the rest of the world — perhaps in the form of a drug that can restore mitochondria function and lipid balance in the degenerating brain.
“Hopefully, with my little contribution, Alzheimer’s can be cured or slowed down sooner. That would be a big relief for patients and their families,” he said. “If you contribute to a better understanding of disease etiology, that’s the most rewarding thing — to make a real, meaningful and impactful contribution to the field.”
Our Experts
Fei Yin, PhD
Associate Professor, Pharmacology, College of Medicine – Tucson
Assistant Director of Translational Neuroscience, Center for Innovation in Brain Science
Contact
Gloria Bloomer
Center for Innovation in Brain Science
520-626-4164
gbloomer@arizona.edu

