Insights: Vaccines, variants and COVID-19 trends
Immunologist Deepta Bhattacharya discusses the newest COVID-19 vaccines and what the future might hold for COVID-19 immunizations.
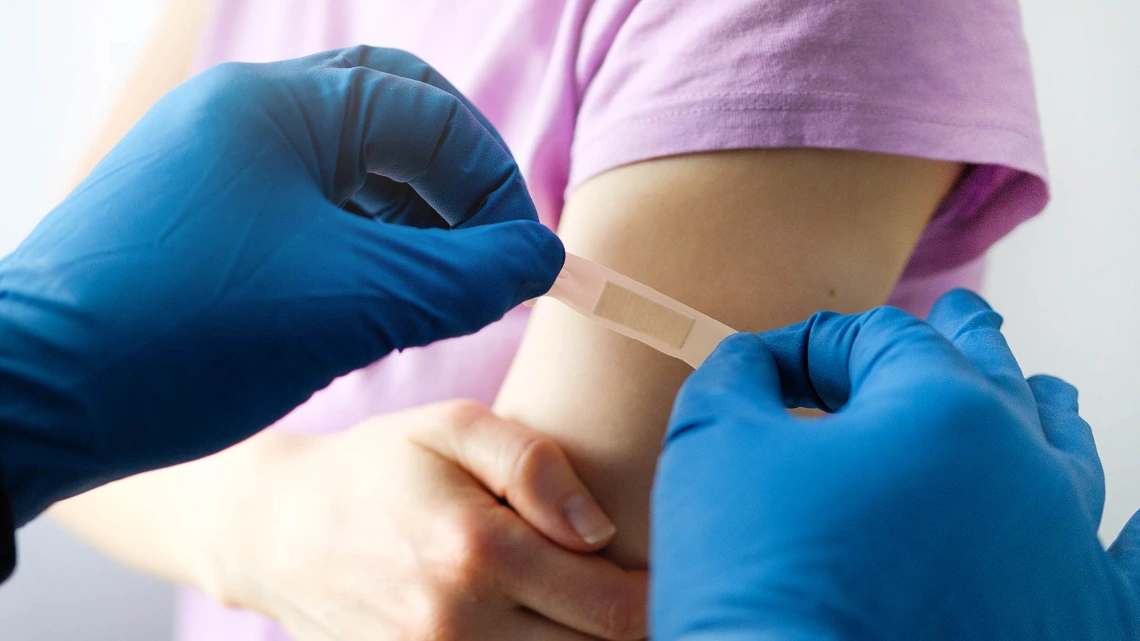
A recent upswing in COVID-19 cases in the U.S. increased conversations about the newest COVID-19 vaccine, which targets the XBB variant.
COVID-19 hospitalizations in the United States were on the rise for weeks heading into mid-September, right as updated COVID-19 vaccines from Pfizer and Moderna started to be distributed across the country.
The U.S. Food and Drug Administration has approved, for emergency use, new COVID-19 vaccines formulated to target circulating variants. The Centers for Disease Control and Prevention is recommending the updated vaccines for everyone 6 months and older to protect against serious illness.

In his lab at the College of Medicine – Tucson’s Department of Immunobiology, Deepta Bhattacharya, PhD, studies both stem cells and antibody responses to infections and vaccines.
Deepta Bhattacharya, PhD, professor in the Department of Immunobiology at the University of Arizona College of Medicine – Tucson, was studying antibody responses to viral infections and vaccines for many years before the pandemic made headlines. He was instrumental in the development of one of the world’s most accurate COVID-19 antibody tests, which is a critical tool for diagnoses and research.
In a recent interview, Bhattacharya, who sits on the advisory council for the UArizona Health Sciences Center for Advanced Molecular and Immunological Therapies and is a member of the BIO5 Institute, answers some of the most common questions surrounding the latest COVID-19 news.
Q: There are concerns about a recent uptick in hospitalizations. How would you describe the current situation?
Bhattacharya: An uptick in respiratory viruses and associated illnesses is to be expected as we head into the fall season. But there is no reason to think we will go back to those horrible days in 2020. Almost everyone now has some form of immunity either from prior vaccinations, infections, or a combination of both. And even though the virus is still evolving and COVID-19 is unfortunately here to stay, there is no reason to think that these new variants will make people as sick as before when they had no immunity.
I would recommend everyone 6 months or older get the new shot, as it is a much preferable experience than the alternative of getting sick. However, it is not as much of an urgent recommendation as it was before for most people. It would be very prudent for the elderly, immunocompromised and people who skipped the last booster to get this updated shot.
Q: How are these updated vaccines different from the last one?
Bhattacharya: These shots use the same mRNA technology as the others, but the difference is in what is being targeted. Last year’s updated vaccine was bivalent, meaning it offered protection against both the original SARS-CoV-2 virus and certain Omicron variants. The new vaccines being offered by Pfizer BioNTech and Moderna have been updated to match the sequence and mutations of the XBB variant, which was the predominant variant earlier this year. Novavax has a protein-based vaccine I anticipate will also get approved soon, and it is also targeting the XBB variant.
The idea is that the closer you get the vaccines to match the current variant in circulation, the better they are going to work. This is the same strategy behind the seasonal flu shot that has been used for decades.
Q: Will COVID-19 vaccinations eventually be recommended annually, like the flu shot?
Bhattacharya: I think for the next few years, at least, that is the periodicity to expect. However, there are a lot of researchers and startup companies that are trying to make a universal COVID-19 vaccine where they try to cover all of the key possibilities of what the virus may do next. And so far, the initial data looks pretty promising. So, it is possible in the next five years or so we might have the option of getting that shot and won’t necessarily need to fall into this seasonal pattern.
Q: Is there anything new about the recommendation for children as young as 6 months to be vaccinated?
Bhattacharya: No, it is not really any different from what the FDA has already approved. The mRNA vaccines had already been approved for 6 months and older. The only difference is that children will have better protection now, because the vaccine has been updated to best match what is currently going around. If I had a kid that young, I would want them to get this updated vaccine.
Q: What would you say to people who are worried about the safety of these vaccines, or are worried about the safety of getting repeated COVID-19 vaccines?
Bhattacharya: The mRNA vaccines were already proven safe when they were first rolled out, and considering billions of shots have been administered at this point, I think we have a pretty good handle on the safety profile of this technology. You might not feel great the day you get the shot, but it is a much better experience than being sick with COVID-19.
When it comes to being worried about revving up your immune system with vaccines every year, all I can say is we have good data on this. There is no evidence of any negative consequence of getting the flu shot year after year, for example. You have to remember, your immune system is constantly fighting off and bouncing back from viral infections. A vaccine is a drop in the bucket compared to what your immune system is exposed to in a year. The immune system is pretty good at dealing with things that come its way, and there is no reason to think it would be markedly worse at dealing with vaccines.
Extras
A New Era: Creating Defenses Against Disease After COVID-19
What We Know About the Omicron Variant and Vaccine Efficacy
What We Know About the Delta Variant and Vaccine Protection
What You Need to Know About a COVID-19 Vaccine
UArizona Health Sciences Study Shows SARS-CoV-2 Antibodies Provide Lasting Immunity
UArizona Partners with State to Provide COVID-19 Antibody Tests
Our Experts
Deepta Bhattacharya, PhD
Professor, Department of Immunobiology, College of Medicine – Tucson
Professor, Department of Surgery, College of Medicine – Tucson
Member, BIO5 Institute
Member, UArizona Cancer Center
Contact
Brian Brennan
UArizona Health Sciences
520-621-3510
brianbrennan@arizona.edu

